Prof Ting XIE
PhD in Molecular Biology and Biochemistry
Kerry Holdings Professor of Science
Chair Professor, Division of Life Science
Director of Biomedical Research Institute, HKUST-Peking University Shenzhen Medical Center
Director of Center for Tissue Regeneration and Engineering
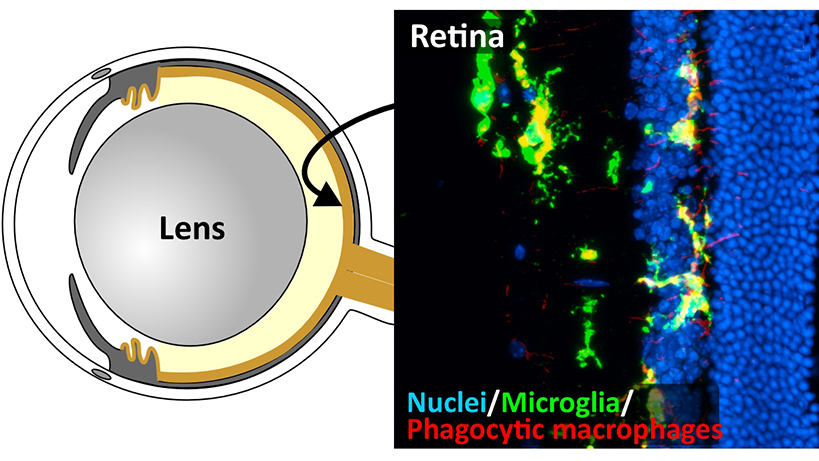
Glaucoma is the second most common cause of blindness worldwide due to irreversible loss of retinal ganglion cells (RGCs), the neurons transmitting visual signal from the eye to the visual cortex. Currently, 80 million people are estimated to have glaucoma worldwide, and the number is expected to continue to rise as the world population continues to increase life expectancy since glaucoma is an age-related disease. High intraocular pressure (IOP) is a major risk of glaucoma, but how high IOP and old age synergize to drive glaucoma pathogenesis remains a mystery. This study is specifically designed to address this outstanding question using a combination of a Zmpste24 knockout (Zmpste24-/-) premature aging mouse model and a silicone oil (SO)-induced high IOP glaucoma model. A homozygous mutation in Zmpste24, which encodes an enzyme processing nuclear lamin A protein, causes premature aging in both mice and humans. In this study, we will utilize electroretinography (ERG) and the optomotor response behavior test to evaluate visual functions, and immunohistochemistry staining to investigate RGC survival and the structural changes of retinal blood vessels and microglia, which accompany the RGC loss. First, we will investigate how the Zmpste24-/- mutation (aging) alone affects visual functions, the RGC survival and retinal blood vessels and microglia at the age of one, three and six months (most of these mutant mice die at 6 months due to aging-associated symptoms, which are roughly equivalent to 80-year-old humans). Then, we will investigate how IOP affects the visual functions of the premature aging mice, which eyes are injected by SO into their anterior chamber to elevate IOP. RNA-sequencing will be applied to uncover the age- and high IOP-related gene expression changes in the retina, while mass spectrometry will be utilized to identify age-related and high IOP-related protein changes in the aqueous humor (AH). Finally, we will use SO-injected naturally aged 1-, 1.5- and 2-year-old mice to further verify the age- and high IOP-related gene expression changes in the retina and protein changes in the AH. The findings from this proposed study will surely provide some novel insight into how aging corroborate with high IOP to drive glaucoma pathogenesis. The long-term goal of this proposed project will be to seek further funding support from Hong Kong government agencies and other sources to develop treatments for hypertension glaucoma in collaboration with the Eye Center of the Chinese University of Hong Kong.